S P D F Orbitals Electron Configuration Definition

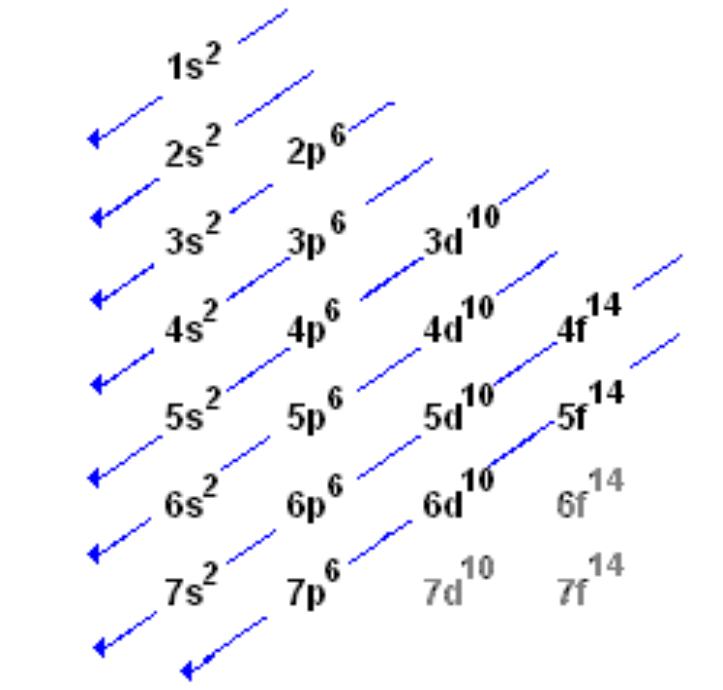
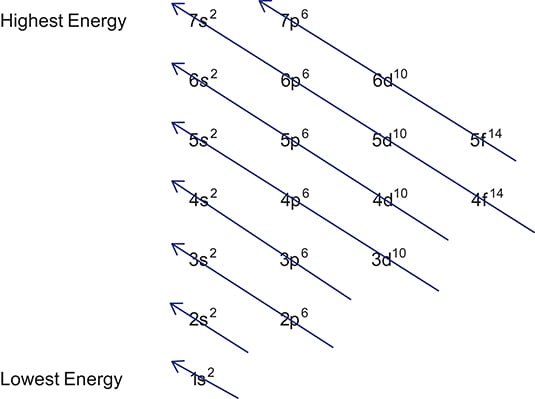
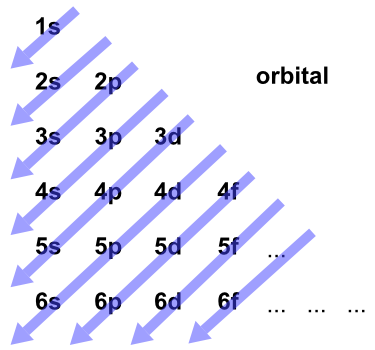
There are more orbitals within f than d and so on.
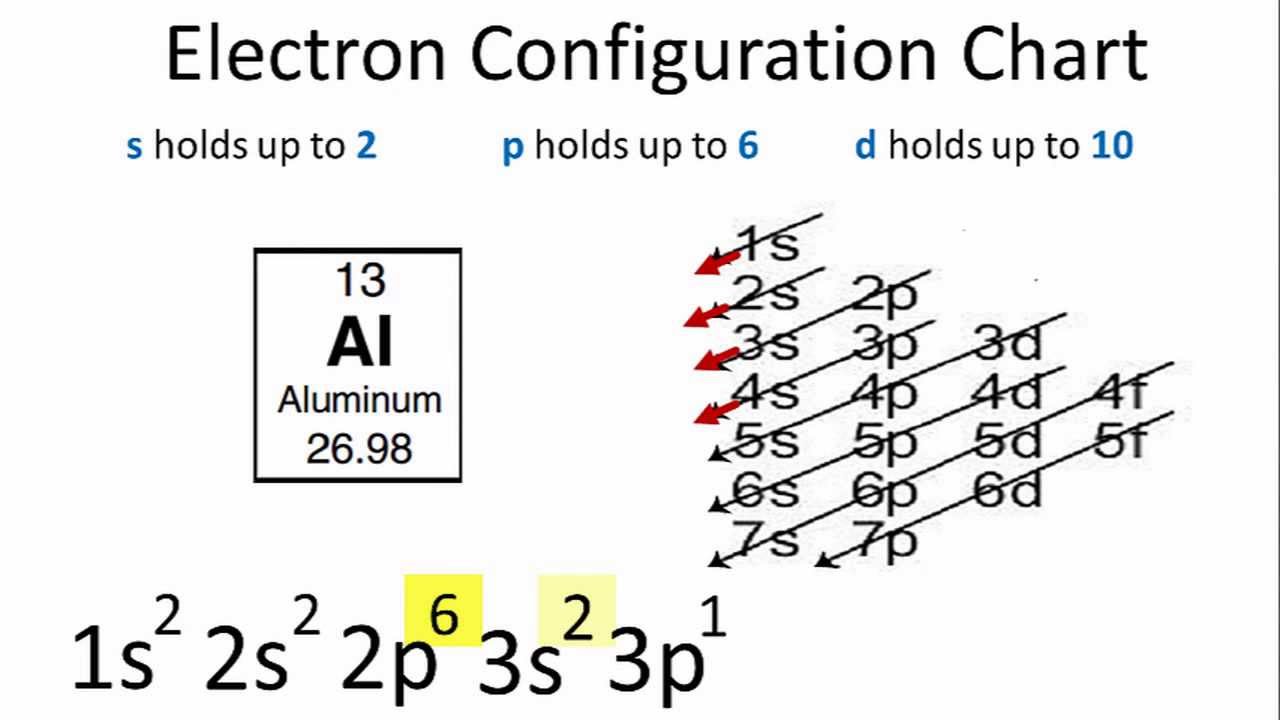
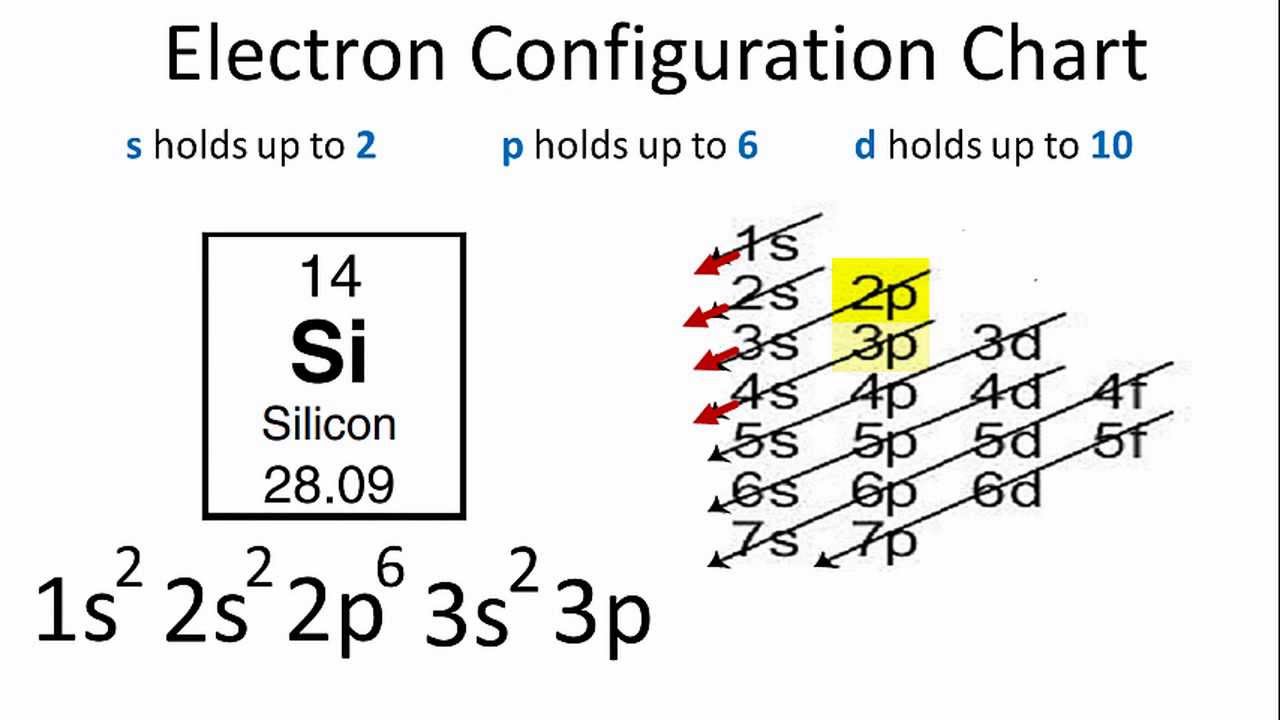
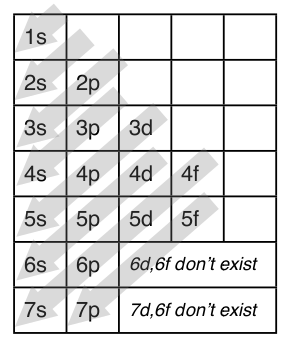
S p d f orbitals electron configuration definition. The smallest sphere is 1s. Electron configuration was first conceived under the bohr model of the atom and it is still common to speak of shells and subshells despite the advances in understanding of the quantum mechanical nature of electrons. P can hold 6 electrons. Within the sphere there are shells in which an electron is more likely to be found at any given time.
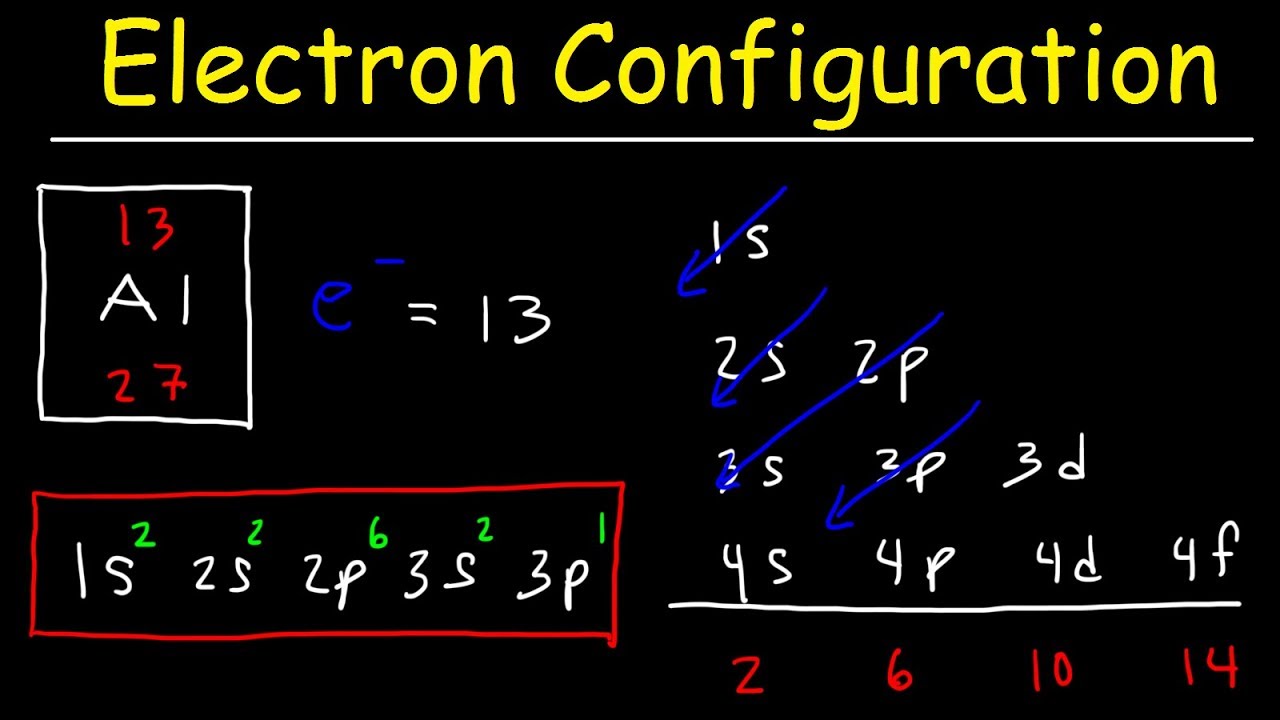
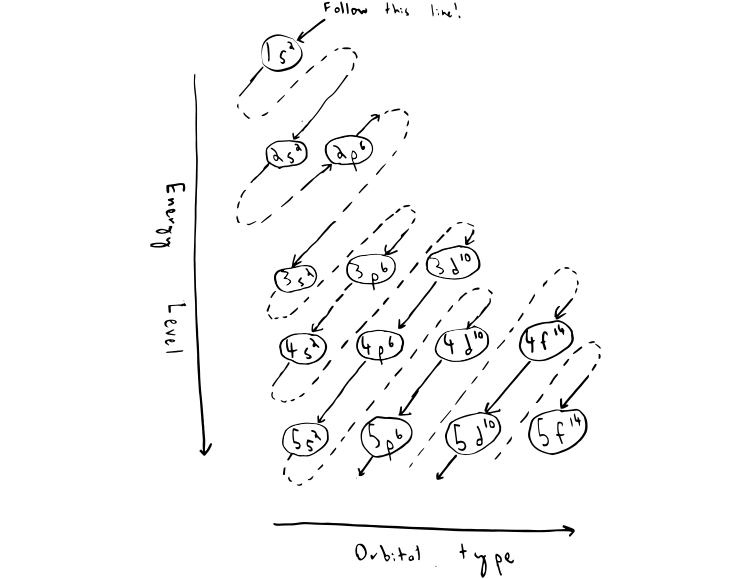
If you were to take the electron configuration of calcium. The orbitals are of 4 types. Therefore the s p d and f subshells can accommodate a maximum of 2 6 10 and 14 electrons respectively. The letters and words refer to the visual impression left by the fine structure of the spectral lines which occurs due to the first relativistic corrections especially the spin orbital interaction.
We call this shape the 95 contour. There can be two electrons within an s orbital p orbital or d orbital. The maximum number of electrons that can be accommodated by a subshell is given by the formula 2 2l 1. The letters s p d and f were assigned for historical reasons that need not concern us.
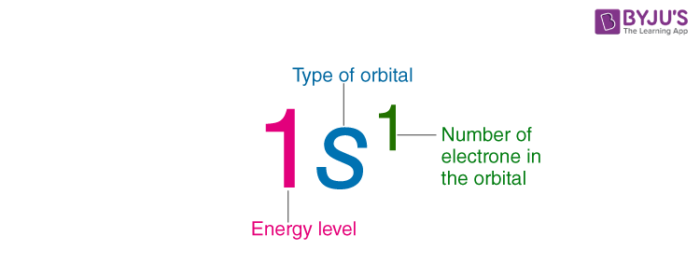
The s orbital is a sphere around the atomic nucleus. All we have to do is remember the shapes that correspond to each letter. The subshells correspond to l 0 l 1 l 2 and l 3 and are named the s p d and f subshells respectively. An electron shell is the set of allowed states that share the same principal quantum number n the number before the letter in the orbital label that electrons may occupy.
Since an electron can theoretically occupy all space it is impossible to draw an orbital. F can hold 14 electrons. That means that for d orbitals to exist there has to be an s orbital with a higher energy level that has at least one electron in it. They are named s p d f the s p d and f stand for sharp principal diffuse and fundamental respectively.
The simple names s orbital p orbital d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ 0 1 2 and 3 respectively. S can hold 2 electrons. All we can do is draw a shape that will include the electron most of the time say 95 of the time. These names together with the value of n are used to describe the electron configurations of atoms.